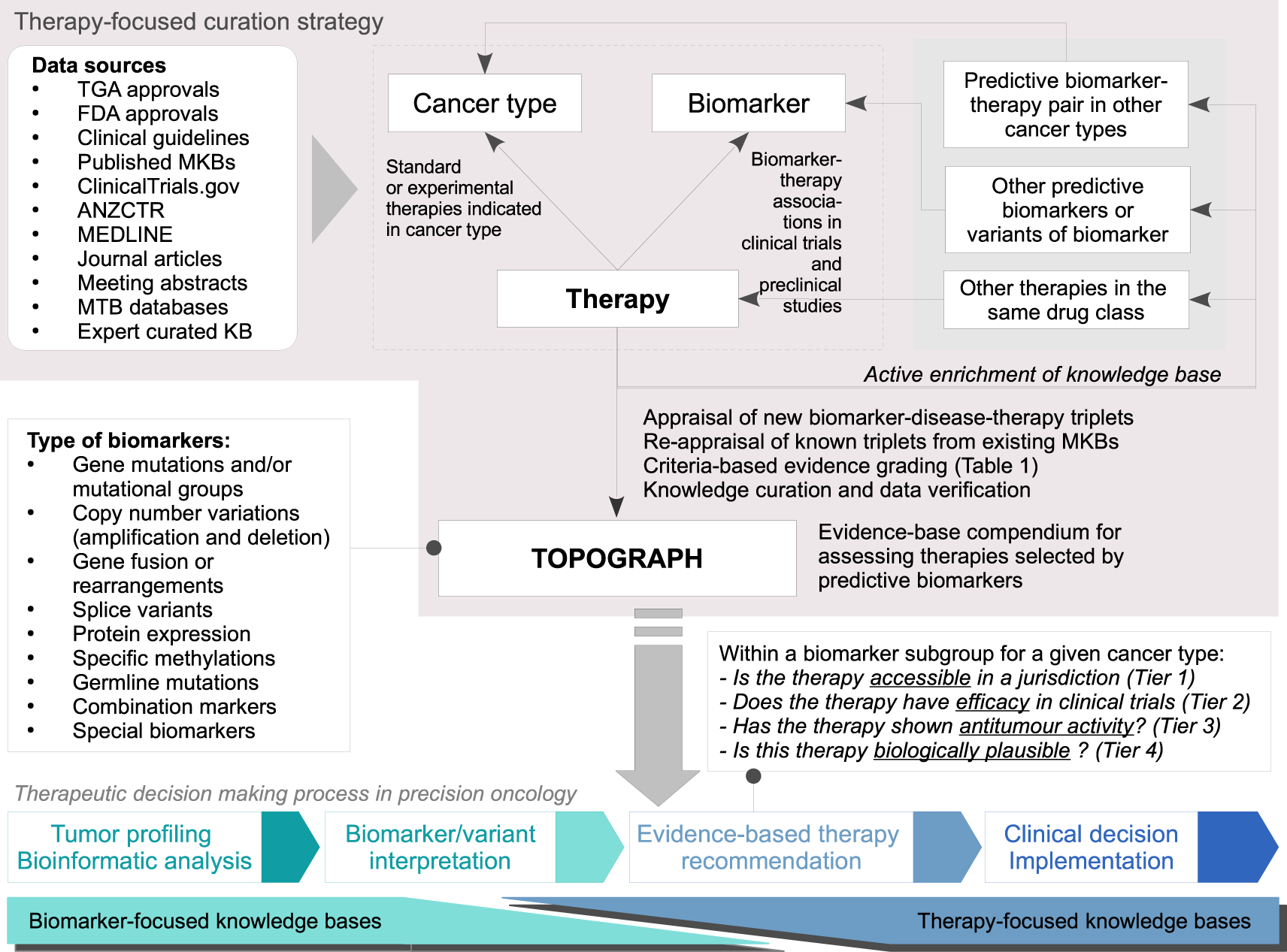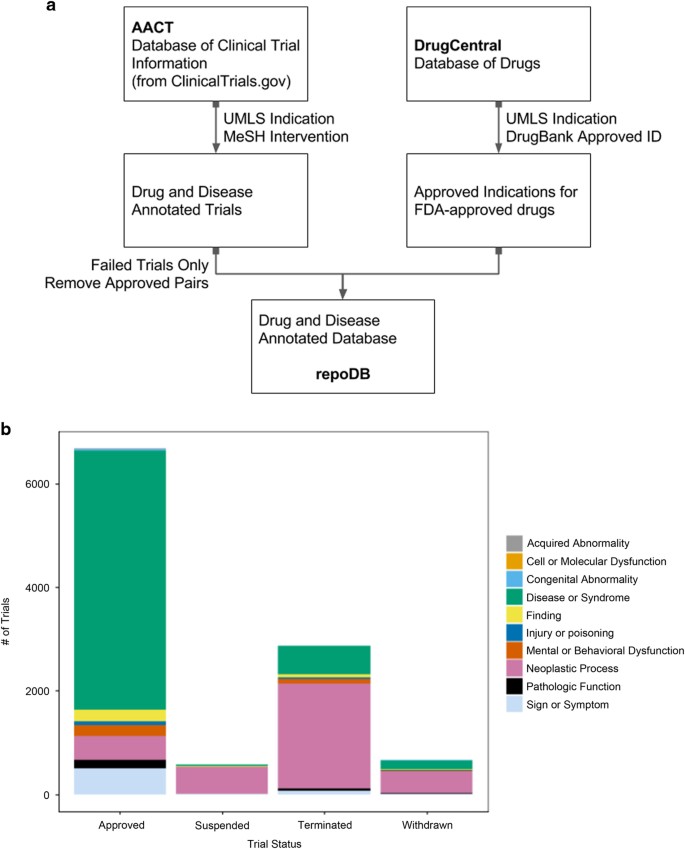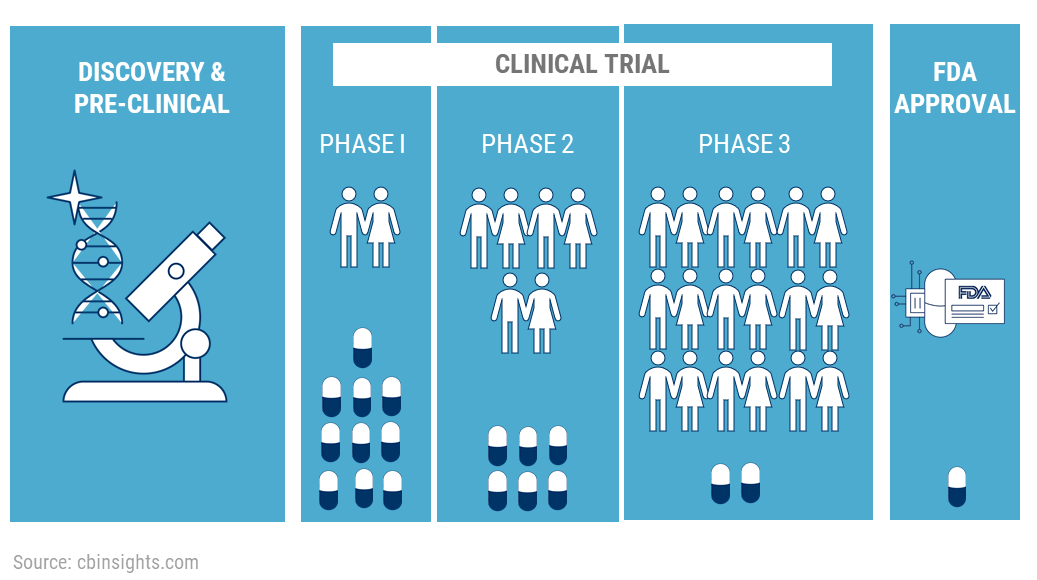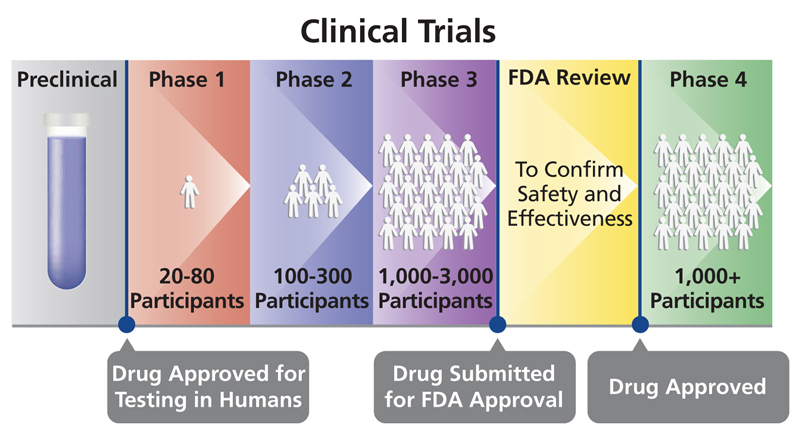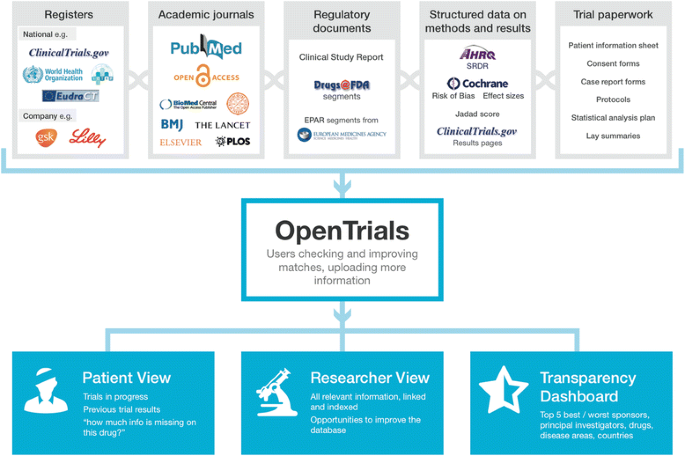
OpenTrials: towards a collaborative open database of all available information on all clinical trials | Trials | Full Text
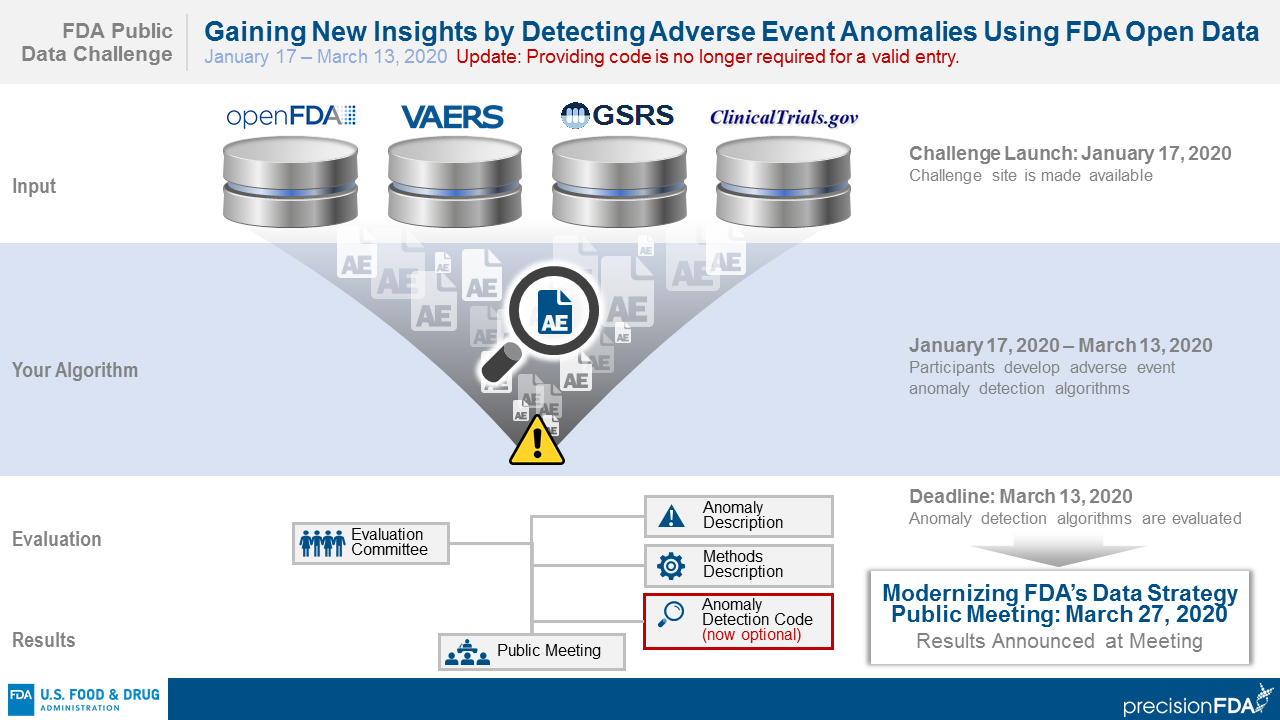
Gaining New Insights by Detecting Adverse Event Anomalies Using FDA Open Data - PrecisionFDA Challenge

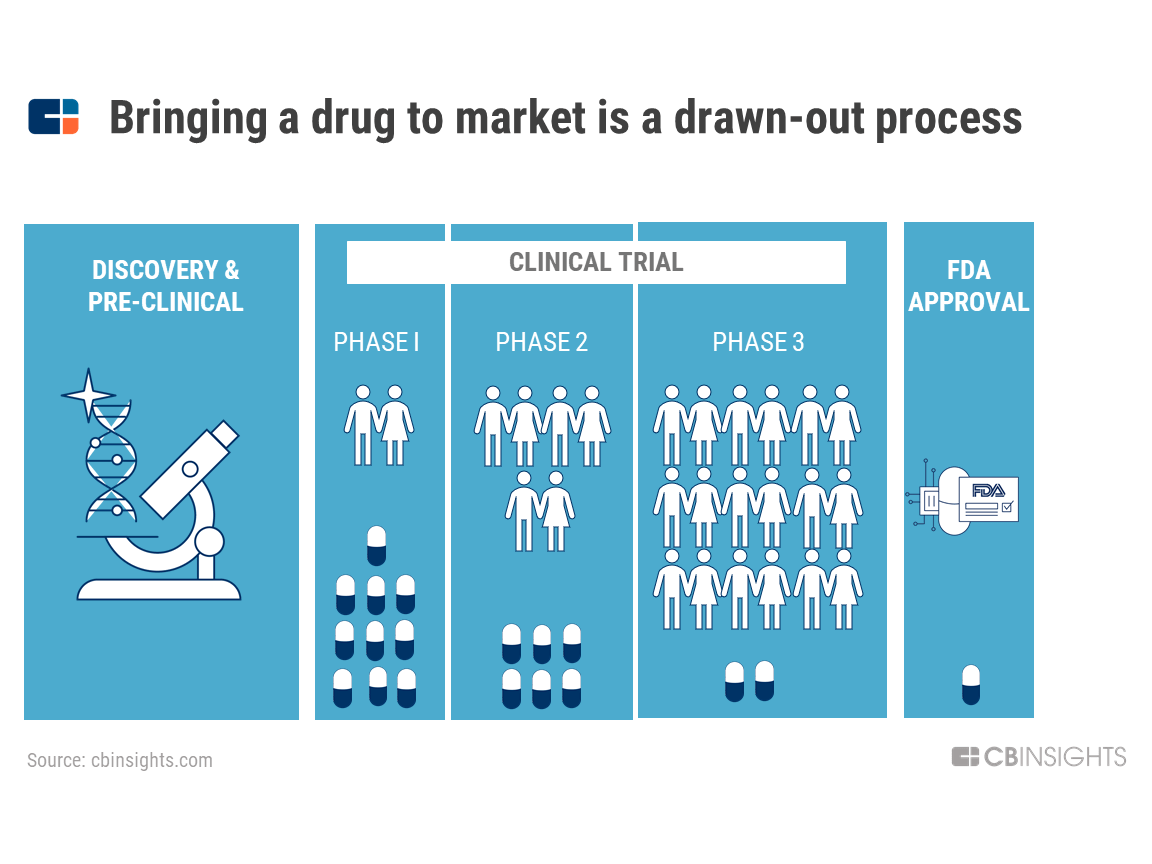
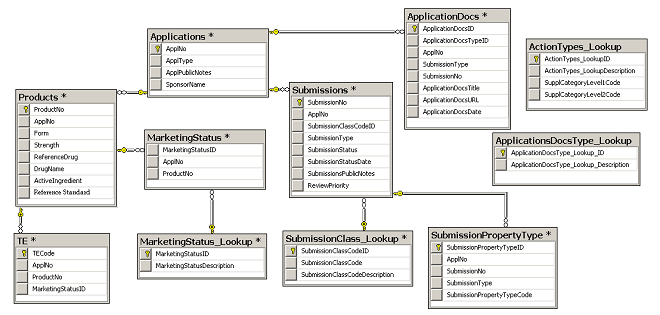
Clinical Trial Transparency: Ensuring Compliance with the New FDA Guidelines | BrackenData Clinical Trial Intelligence

Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: A safety analysis of clinical trials and FDA pharmacovigilance database - eClinicalMedicine