Formedix and CDISC Join Forces to Provide Free Access to Electronic Case Report Forms for Accelerated Clinical Trial Set-Up | Technology Networks

Sections of CDISC ODM with study metadata information left-hand side... | Download Scientific Diagram

Understanding of data capturing in standardized CDISC-SDTM format – Clinical trials and CRO centric systems

A pragmatic method for transforming clinical research data from the research electronic data capture “REDCap” to Clinical Data Interchange Standards Consortium (CDISC) Study Data Tabulation Model (SDTM): Development and evaluation of REDCap2SDTM -

OpenClinica becomes Gold Member with the Clinical Data Interchange Standards Consortium (CDISC) » OpenClinica




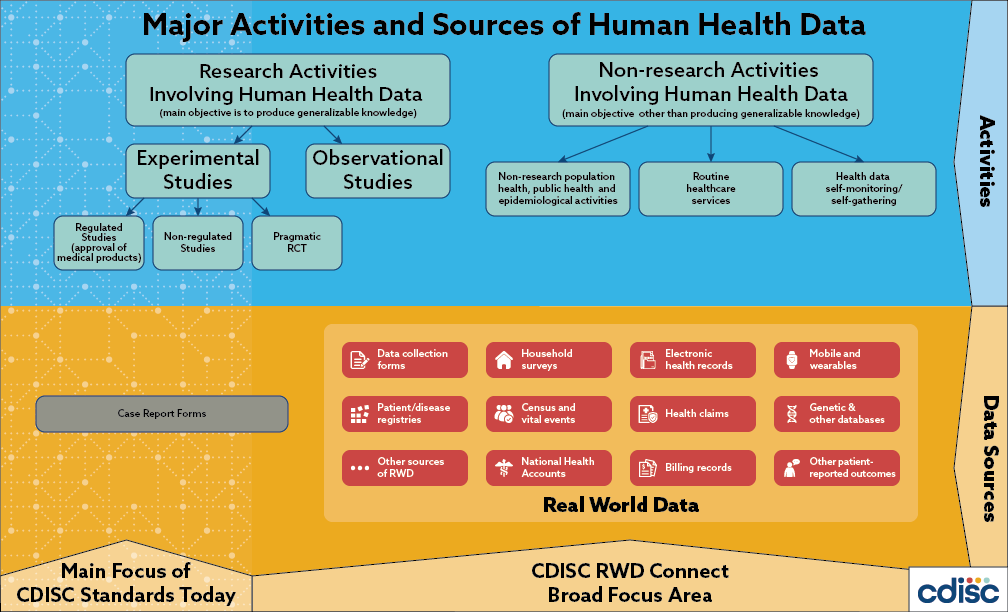


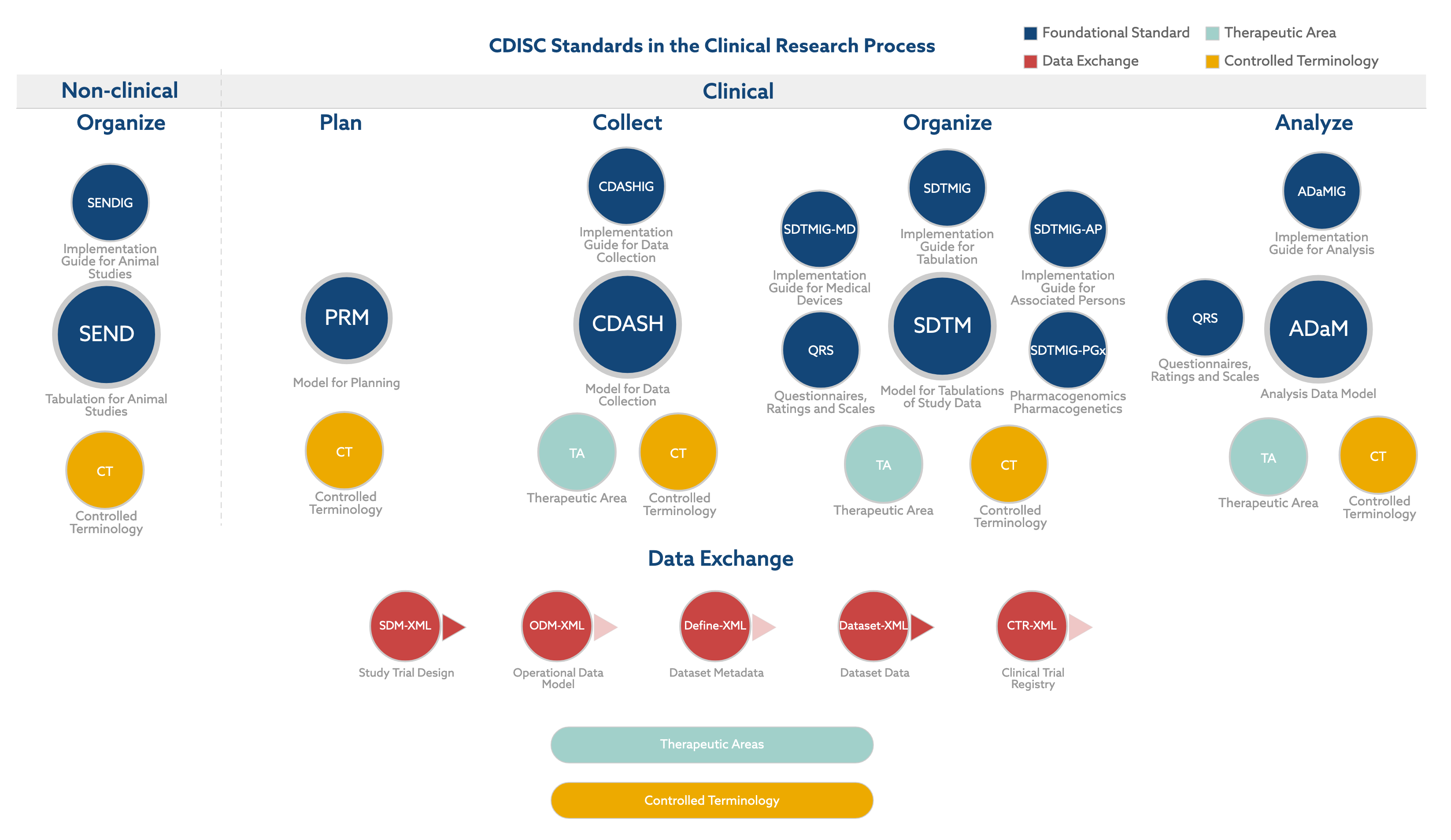
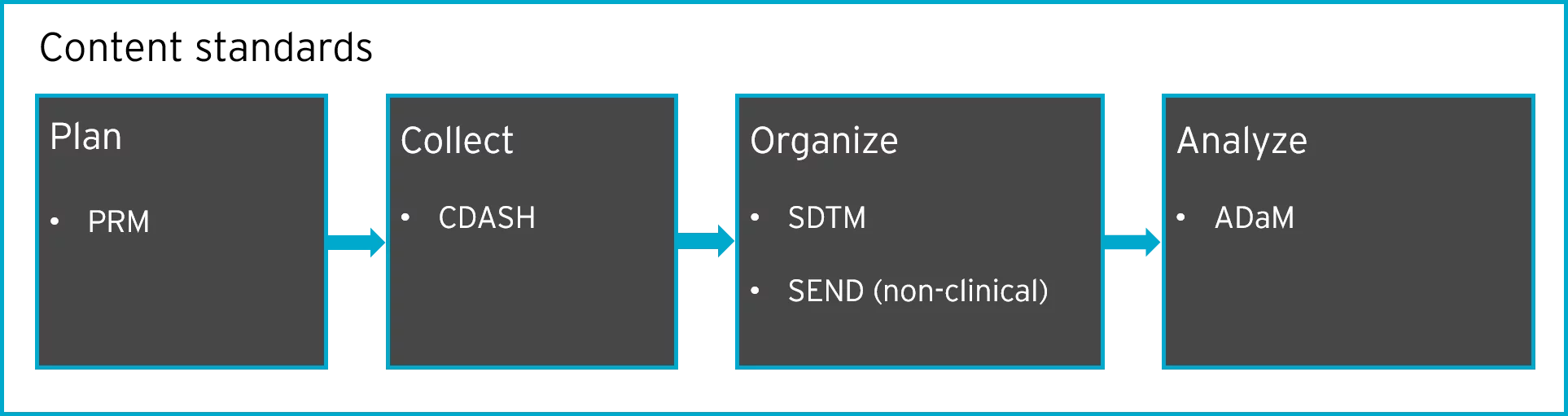
.png)